
Rust is not just a deposit, but the result of a complex chemical reactionthat literally eats the metal from the inside out. This process is officially called oxidationand it occurs almost everywhere where iron is present. Understanding the causes of red stains helps to protect machinery, tools and building structures in time.
The main culprits of corrosion
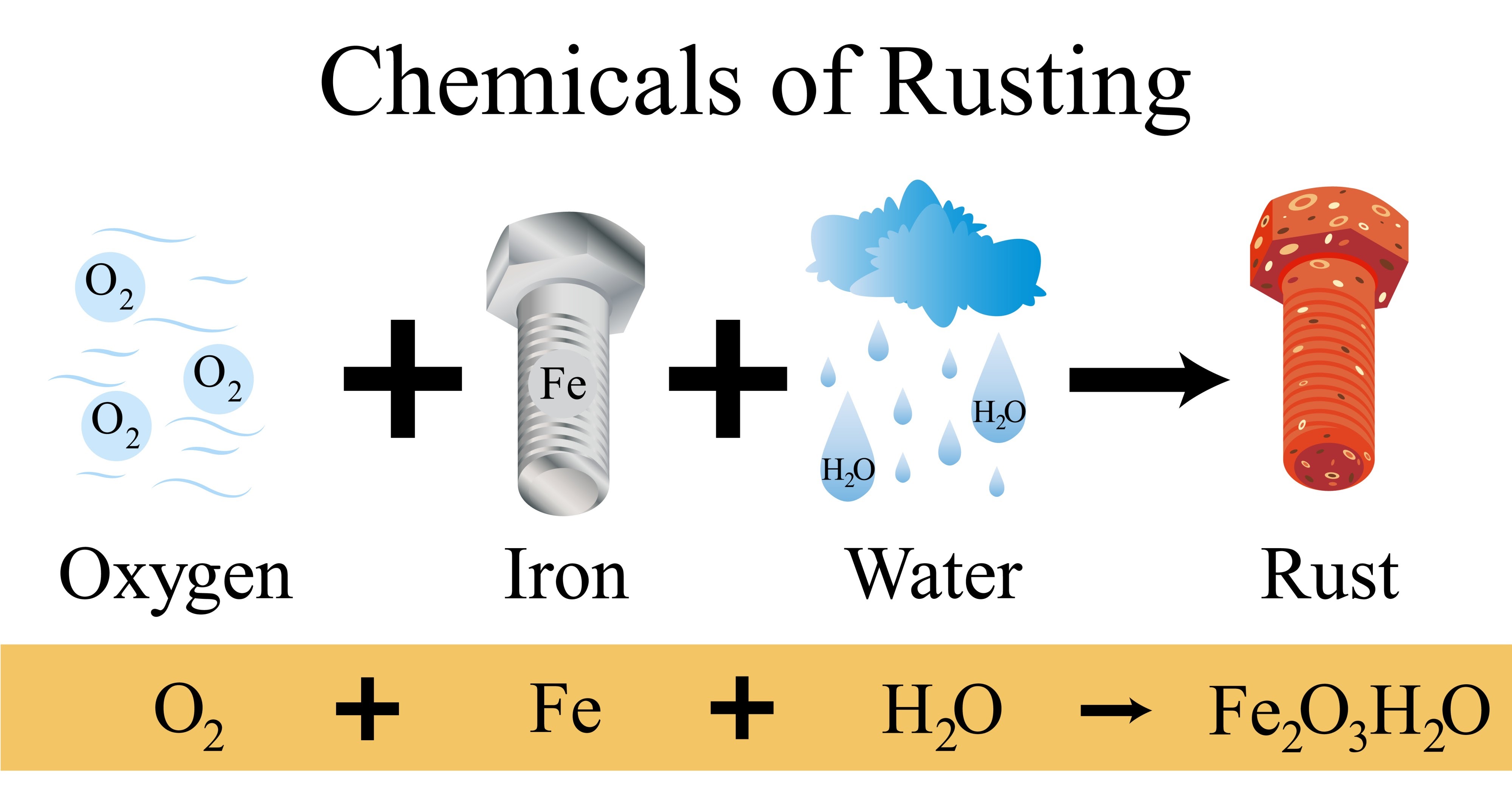
The basic mechanism of rusting is triggered by the participation of three components: iron, water and oxygen. Without any of these elements, the reaction is either slowed down or becomes impossible.
-
-
Air humidity: Even if the metal is not exposed to a direct stream of water, microscopic droplets of condensation settle on the surface, becoming an ideal medium for reaction.
-
Oxygen: It acts as an oxidizing agent that interacts with metal atoms.
-
Electrolytes: Salt, road chemicals and acids in sludge work as catalysts. They accelerate the movement of electrons, which makes rust spread many times faster.
-
Shutterstock
Chemical process in simple words
When iron comes in contact with water and oxygen, iron is formed hydrated iron oxide. Unlike patina on copper or oxide film on aluminum, rust has a porous structure. It does not protect the metal, but instead absorbs moisture, allowing corrosion to penetrate deeper into the material structure until the part is completely destroyed.
External risk factors
The rate of metal degradation is highly dependent on the environment. There are conditions under which corrosion becomes aggressive.
-
Temperature fluctuations: Abrupt changes in heat and cold lead to condensation in hidden cavities.
-
Marine climate: The high salt content in the air makes the oxidation process almost instantaneous.
-
Surface contamination: Dirt and dust keep moisture on the metal longer, creating a “wet compression” effect.
Preventing deterioration is helped by timely insulation surfaces from contact with the outside environment. The use of protective coatings, galvanization and humidity control remain the most effective ways to preserve the integrity of metal products for years to come. Regular inspection can detect the first signs of oxidation before structural changes become irreversible.




